 |
 |
| Tuberc Respir Dis > Epub ahead of print |
|
Abstract
Background
Methods
Results
Notes
AuthorsŌĆÖ Contributions
Conceptualization: Yoon HY. Methodology: Yoon HY. Formal analysis: Seok J. Data curation: Seok J. Software: Seok J. Validation: all authors. Investigation: Seok J. Writing - original draft preparation: all authors. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Fig.┬Ā1.
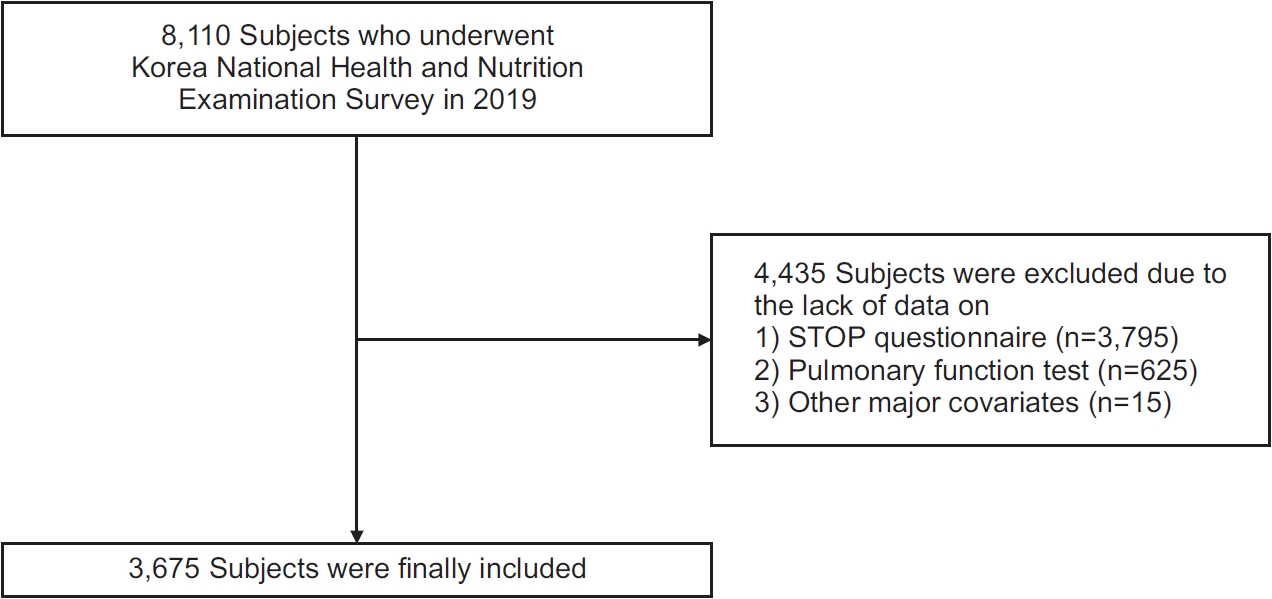
Fig.┬Ā2.

Table┬Ā1.
Table┬Ā2.
Table┬Ā3.
Values are presented as number (%), mean┬▒standard deviation, or median (interquartile range).
FVC: forced vital capacity; FEV1: forced expiratory volume per 1 second; FEF25-75: forced expiratory flow between 25% and 75% of vital capacity; PEF: peak expiratory flow; PRISm: preserved ratio impaired spirometry; STOP: Snoring, Tiredness during daytime, Observed apnea, and high blood Pressure.
Table┬Ā4.
The r-values were calculated using PearsonŌĆÖs correlation coefficients.
STOP-Bang: Snoring, Tiredness during daytime, Observed apnea, and high blood Pressure-Body mass index, Age, Neck circumference, Gender; FVC: forced vital capacity; FEV1: forced expiratory volume per 1 second; FEF25-75: forced expiratory flow between 25% and 75% of vital capacity; PEF: peak expiratory flow; BMI: body mass index.
Table┬Ā5.
| Variable |
Univariate |
Multivariable* |
||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Lung disease typeŌĆĀ | ||||
| ŌĆāNormal | 1.000 (reference) | 1.000 (reference) | ||
| ŌĆāObstructive | 0.707 (0.565-0.886) | 0.003 | 1.166 (0.862-1.575) | 0.319 |
| ŌĆāPRISm | 1.469 (1.107-1.950) | 0.008 | 1.145 (0.864-1.516) | 0.346 |
| FVC, % predicted | 0.968 (0.958-0.972) | <0.001 | 0.999 (0.990-1.008) | 0.784 |
| FVC <80% predicted | 2.292 (1.906-2.757) | <0.001 | 1.109 (0.872-1.408) | 0.394 |
| FEV1, % predicted | 0.980 (0.974-0.9860 | <0.001 | 0.994 (0.869-1.001) | 0.113 |
| FEV1 <80% predicted | 1.512 (1.253-1.826) | <0.001 | 1.090 (0.862-1.378) | 0.473 |
| FEV1/FVC | 0.959 (0.949-0.970) | <0.001 | 0.991 (0.976-1.007) | 0.257 |
| FEV1/FVC <0.7ŌĆĪ | 1.919 (1.529-2.408) | <0.001 | 1.129 (0.842-1.515) | 0.417 |
| FEF25-75 | 1.032 (0.941-1.132) | 0.504 | 0.958 (0.840-1.094) | 0.527 |
| PEF | 1.251 (1.198-1.306) | <0.001 | 1.028 (0.957-1.103) | 0.450 |
| Cough | 1.958 (1.257-3.050) | 0.003 | 2.413 (1.383-4.213) | 0.002 |
| Sputum | 2.345 (1.620-3.394) | <0.001 | 1.868 (1.166-2.992) | 0.009 |
* Multivariable model adjusted for age, sex, body mass index, history of diabetes mellitus and hypertension, smoking status, and household income;
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 337 View
- 22 Download
- ORCID iDs
-
Jinwoo Seok

https://orcid.org/0000-0002-7776-2797Hee-Young Yoon

https://orcid.org/0000-0001-9852-0036 - Related articles


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Data Sharing Statement
Data Sharing Statement Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



