 |
 |
| Tuberc Respir Dis > Volume 87(2); 2024 > Article |
|
Abstract
Background
Methods
Results
Notes
Authors’ Contributions
Conceptualization: De S. Methodology: Sahu D, De S. Formal analysis: Rath AK, De S. Data curation: Rath AK, Sahu D. Software: Rath AK, De S. Validation: Rath AK, De S. Investigation: all authors. Writing - original draft preparation: all authors. Writing - review and editing: all authors. Approval of final manuscript: all authors.
Acknowledgments
Fig. 1.

Fig. 2.
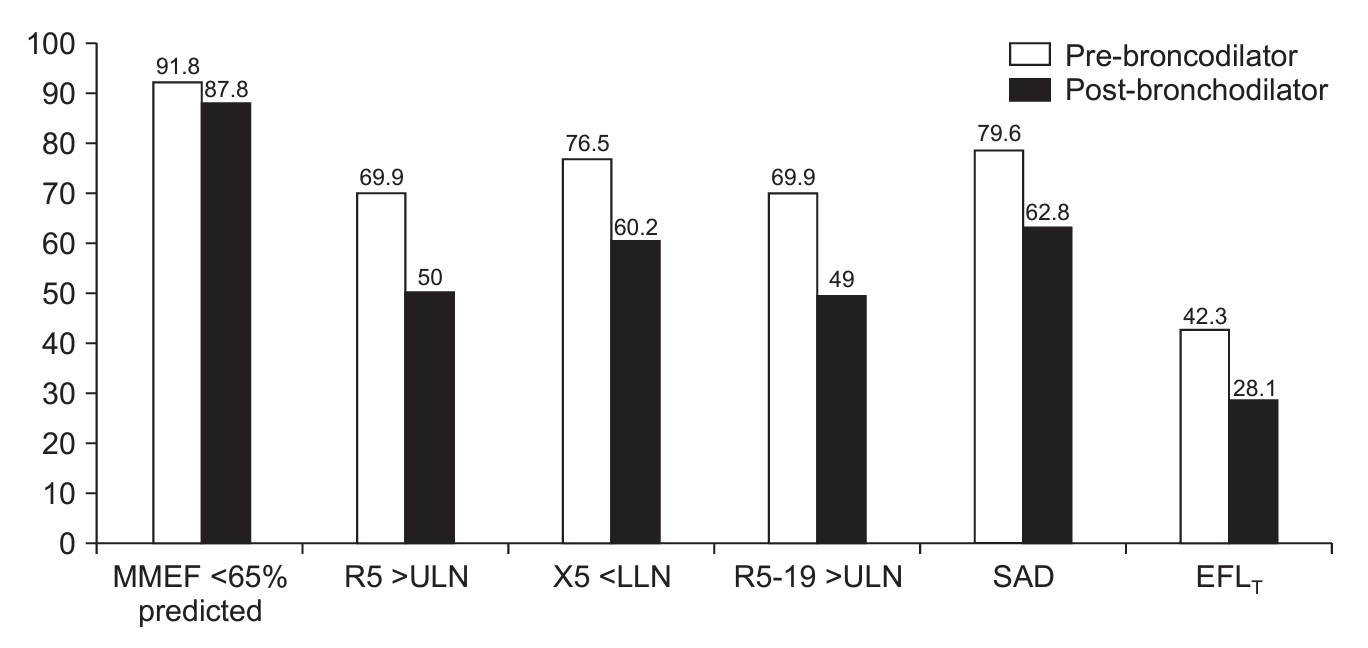
Table 1.
| Variable | Total (n=196) | GOLD grade 1 (n=28) | GOLD grade 2 (n=88) | GOLD grade 3 (n=65) | GOLD grade 4 (n=15) | p-value |
|---|---|---|---|---|---|---|
| Age, yr | 63.3±8.4 | 64.5±5.2 | 63.4±9.4 | 63.6±7.9 | 58.7±9.2 | 0.17 |
| Male sex | 188 (95.9) | 25 (89.3) | 86 (99.7) | 62 (95.4) | 15 (100) | 0.21 |
| BMI, kg/m2 | 22.5±4.8 | 23.7±4.1 | 22.4±4.1 | 22.6±5.8 | 20.3±4.9 | 0.19 |
| Smoker | 168 (86.7) | 21 (75.0) | 76 (86.4) | 59 (90.8) | 12 (80.0) | 0.51 |
| Pack-years | 32.9 (15-52) | 36.9 (10.7-56.7) | 26.9(14-55.3) | 40 (17.8-52) | 26.3 (18.3-45.1) | 0.78 |
| CAT score | 14.5±6.1 | 10.7±4.6 | 14.4±6.6 | 15.9±5.4 | 15.5±5.9 | <0.01* |
| CAT score ≥10 | 155 (79.1) | 17 (60.7) | 69 (78.4) | 58 (89.2) | 11 (73.3) | 0.02* |
| Absolute eosinophil count | 120 (30-230) | 120 (30-230) | 120 (20-230) | 120 (30-235) | 110 (60-210) | 0.83 |
| GOLD group A | 37 (18.9) | 11 (39.3) | 17 (19.3) | 6 (9.2) | 3 (20.0) | 0.05 |
| GOLD group B | 107 (54.6) | 13 (46.4) | 48 (54.5) | 39 (60.0) | 7 (46.7) | |
| GOLD group E | 52 (26.5) | 4 (14.3) | 23 (26.1) | 20 (30.8) | 5 (33.3) | |
| Post-BD FEV1, L | 1.26±0.49 | 1.96±0.51 | 1.41±0.31 | 0.91±0.16 | 0.60±0.11 | <0.01* |
| Post-BD FEV1, % predicted | 56.4±20.2 | 91.5±11.6 | 62.1±8.1 | 40.7±6.0 | 25.2±2.61 | <0.01* |
| Post-BD FEV1/FVC | 53.6±10.3 | 65.6±6.3 | 56.6±7.4 | 48.0±7.7 | 37.1±3.74 | <0.01* |
| Post-BD MMEF, % of predicted | 38.3±20.5 | 72.7±18.9 | 41.3±12.7 | 24.9±7.7 | 13.7±2.7 | <0.01* |
| Post-BD MMEF <65% of predicted | 172 (87.8) | 9 (32.1) | 83 (94.3) | 65 (100) | 15 (100) | <0.01* |
| Post-BD sRaw, kPa/sec | 2.77±2.34 | 1.07±0.55 | 1.88±1.26 | 3.50±1.38 | 8.02±3.75 | <0.01* |
| Post-BD TLC, L | 5.6±1.05 | 5.42±0.96 | 5.63±1.08 | 5.49±1.03 | 6.02±1.13 | 0.25 |
| Post-BD RV/TLC, % of predicted | 148.3±29.7 | 114.1±20.0 | 141.1±19.1 | 161.3±21.2 | 197.9±33.9 | <0.01* |
| DLCOc, mmol/min/kPa | 5.11±1.56 | 5.81±1.36 | 5.25±1.67 | 4.82±1.29 | 4.15±1.77 | <0.01* |
| kCo, % of predicted | 85.8±22.71 | 88.1±21.0 | 84.9±21.5 | 88.3±23.6 | 74.7±27.5 | 0.21 |
| Post-BD R5, cm H2O/L/sec | 5.12±1.73 | 3.71±1.32 | 4.65±1.56 | 6.05±1.52 | 6.45±1.15 | <0.01* |
| Post-BD R5 >ULN | 98 (50.0) | 3 (10.7) | 28 (31.8) | 52 (80.0) | 15 (100) | <0.01* |
| Post-BD X5, cm H2O/L/sec | -3.25±2.32 | -1.33±0.71 | -2.43±1.75 | -4.44±2.05 | -6.41±2.67 | <0.01* |
| Post-BD X5 <LLN | 118 (60.2) | 3 (10.7) | 42 (47.7) | 58 (89.2) | 15 (100) | <0.01* |
| Post-BD ∆X5, cm H2O/L/sec | 2.08±2.56 | 0.15±0.66 | 1.31±2.01 | 3.31±2.54 | 4.87±3.06 | <0.01* |
| Post-BD R19, cm H2O/L/sec | 3.62±1.04 | 3.21±0.99 | 3.47±1.0 | 3.93±1.06 | 3.61±1.04 | <0.01* |
| Post-BD R19 >ULN | 75 (38.5) | 4 (14.3) | 27 (30.7) | 36 (56.3) | 8 (53.3) | <0.01* |
| Post-BD R5-19, cm H2O/L/sec | 1.21±0.99 | 0.38±0.59 | 0.87±0.79 | 1.71±0.80 | 2.63±0.81 | <0.01* |
| Post-BD R5-19 >ULN | 96 (48.9) | 3 (10.7) | 27 (30.7) | 51 (78.5) | 15 (100) | <0.01* |
| Post-BD SAD | 123 (62.8) | 4 (14.3) | 45 (51.1) | 59 (90.8) | 15 (100) | <0.01* |
| Post-BD EFLT | 55 (28.1) | 0 | 12 (13.6) | 33 (50.8) | 10 (66.7) | <0.01* |
| 6MWD, m | 376.0±69.3 | 398.9±64.4 | 385.4±66.3 | 363.0±71.2 | 334.0±64.7 | <0.01* |
| Desaturation during 6MWT, % | 4.4±4.2 | 2.1±2.1 | 4.0±4.2 | 5.3±3.9 | 7.6±5.3 | <0.01* |
| Change in the Borg score after 6MWT | 2.38±1.75 | 1.39±1.29 | 2.23±1.73 | 2.85±1.86 | 3.13±1.25 | <0.01* |
GOLD: Global Initiative for Chronic Obstructive Lung Disease; BMI: body mass index; CAT: COPD assessment test; Post-BD: post-bronchodilator, parameters measured after 400 μg inhalation of salbutamol; FEV1: forced expiratory volume 1 second; FVC: forced vital capacity; MMEF: maximal mid-expiratory flow; sRaw: specific airway resistance; TLC: total lung capacity; RV/TLC: ratio of residual volume to total lung capacity; DLCOc: hemoglobin adjusted diffusing capacity of the lungs for carbon monoxide; kCo: diffusion coefficient for carbon monoxide; R5: respiratory system resistance at 5 Hz; ULN: upper limit of normal; X5: respiratory system reactance at 5 Hz; LLN: lower limit of normal; ΔX5: difference in inspiratory and expiratory reactance at 5 Hz; R19: respiratory system resistance at 19 Hz; R5-19: difference in whole-breath resistance at 5 and 19 Hz; SAD: small airway dysfunction; EFLT: expiratory flow limitation at tidal breath; 6MWD: 6-minute walk distance; 6MWT: 6-minute walk test.
Table 2.
| Variable | GOLD group A (n=37) | GOLD group B (n=107) | GOLD group E (n=52) | p-value |
|---|---|---|---|---|
| Age, yr | 61.9±8.5 | 62.8±7.6 | 65.1±9.8 | 0.16 |
| Post-BD FEV1, % of predicted | 65.22±23.2 | 55.72±19.7 | 51.39±17.1 | <0.01* |
| Post-BD FEV1/FVC | 57.1±10.3 | 54.2±9.9 | 49.6±10.0 | <0.01* |
| Post-BD MMEF, % predicted | 48.9±26.5 | 37.8±19.6 | 31.5±13.8 | <0.01* |
| Post-BD MMEF <65% of predicted | 27 (72.9) | 95 (88.8) | 50 (96.2) | <0.01* |
| Post-BD sRaw, kPa/sec | 2.04±2.11 | 2.89±2.53 | 3.05±1.97 | 0.09 |
| Post-BD TLC, L | 5.5±0.9 | 5.55±1.1 | 5.71±1.2 | 0.57 |
| Post-BD RV/TLC, % of predicted | 133.7±34.2 | 150.8±26.5 | 153.6±29.9 | <0.01* |
| DLCOc, mmol/min/kPa | 5.61±1.21 | 5.19±1.57 | 4.6±1.65 | <0.01* |
| Post-BD R5, cm H2O/L/sec | 4.52±1.81 | 5.22±1.65 | 5.34±1.77 | 0.06 |
| Post-BD R5 >ULN | 12 (32.4) | 57 (53.3) | 29 (55.8) | 0.06 |
| Post-BD X5, cm H2O/L/sec | -2.44±2.0 | -3.29±2.17 | -3.73±2.70 | 0.03* |
| Post-BD X5 <LLN | 14 (37.8) | 68 (63.6) | 36 (69.2) | <0.01* |
| Post-BD ∆X5 | 1.38±2.20 | 2.12±2.44 | 2.51±2.96 | 0.12 |
| Post-BD EFLT | 8 (21.6) | 32 (29.9) | 15 (28.8) | 0.62 |
| Post-BD R19, cm H2O/L/sec | 3.46±1.05 | 3.68±1.04 | 3.60±1.04 | 0.54 |
| Post-BD R19 >ULN | 11 (29.7) | 44 (41.5) | 20 (38.5) | 0.45 |
| Post-BD R5-19, cm H2O/L/sec | 0.69±0.85 | 1.29±0.97 | 1.43±0.99 | <0.01* |
| Post-BD R5-19 >ULN | 10 (27.0) | 56 (52.3) | 30 (57.7) | 0.01* |
| Post-BD SAD | 15 (40.5) | 71 (66.4) | 37 (71.2) | <0.01* |
| 6MWD, m | 405.8±61.2 | 371.4±69.8 | 374.2±69.1 | 0.01* |
| Desaturation during 6MWT, % | 4.1±4 | 4.2±4.1 | 5.2±4.4 | 0.276 |
GOLD: Global Initiative for Chronic Obstructive Lung Disease; Post-BD: post-bronchodilator, parameters measured after 400 μg inhalation of salbutamol; FEV1: forced expiratory volume 1 second; FVC: forced vital capacity; MMEF: maximal mid-expiratory flow; sRaw: specific airway resistance; TLC: total lung capacity; RV/TLC: ratio of residual volume to total lung capacity; DLCOc: hemoglobin adjusted diffusing capacity of the lungs for carbon monoxide; R5: respiratory system resistance at 5 Hz; ULN: upper limit of normal; X5: respiratory system reactance at 5 Hz; LLN: lower limit of normal; ΔX5: difference in inspiratory and expiratory reactance at 5 Hz; EFLT: expiratory flow limitation at tidal breath; R19: respiratory system resistance at 19 Hz; R5-19: difference in whole-breath resistance at 5 and 19 Hz; SAD: small airway dysfunction; 6MWD: 6-minute walk distance; 6MWT: 6-minute walk test.
Table 3.
| Characteristic | COPD without SAD (n=73) | COPD with SAD (n=123) | p-value |
|---|---|---|---|
| Age, yr | 63.7±8 | 63.0±8.7 | 0.59 |
| BMI, kg/m2 | 22.9±3.9 | 22.3±5.3 | 0.38 |
| Smoker | 60 (82.2) | 108 (87.8) | 0.24 |
| CAT score | 12.8±6.6 | 15.5±5.6 | <0.01* |
| GOLD group A | 22 (30.1) | 15 (12.2) | <0.01* |
| GOLD group B | 36 (49.3) | 71 (57.7) | <0.01* |
| GOLD group E | 15 (20.5) | 37 (30.1) | <0.01* |
| Post-BD FEV1, % of the predicted | 72.8±17.8 | 46.6±14.5 | <0.01* |
| Post-BD MMEF <65% of predicted | 53 (72.6) | 119 (96.7) | <0.01* |
| Post-BD sRaw, kPa/sec | 1.49±1.35 | 3.53±2.46 | <0.01* |
| Post-BD TLC, L | 5.55±0.92 | 5.60±1.13 | 0.74 |
| Post-BD RV/TLC, % of predicted | 129±24 | 159.7±26.8 | <0.01* |
| DLCOc, mmol/min/kPa | 5.63±1.54 | 4.8±1.50 | <0.01* |
| Post-BD R5, cm H2O/L/sec | 3.75±1.15 | 5.93±1.49 | <0.01* |
| 6MWD, m | 390.3±57.4 | 367.5±74.4 | 0.02* |
| Desaturation during 6MWT, % | 3.2±4.2 | 5.2±3.9 | <0.01* |
| Change in the Borg score after 6MWT | 1.97±1.74 | 2.63±1.72 | 0.01* |
COPD: chronic obstructive pulmonary disease; SAD: small airway dysfunction; BMI: body mass index; CAT: COPD assessment test; GOLD: Global Initiative for Chronic Obstructive Lung Disease; Post-BD: post-bronchodilator, parameters measured after 400 μg inhalation of salbutamol; FEV1: forced expiratory volume 1 second; MMEF: maximal mid-expiratory flow; sRaw: specific airway resistance; TLC: total lung capacity; RV/TLC: ratio of residual volume to total lung capacity; DLCOc: hemoglobin adjusted diffusing capacity of the lungs for carbon monoxide; R5: respiratory system resistance at 5 Hz; 6MWD: 6-minute walk distance; 6MWT: 6-minute walk test.
Table 4.
| Characteristic | SAD without EFLT (n=68) | SAD with EFLT (n=55) | p-value |
|---|---|---|---|
| Age, yr | 63.1±7.5 | 62.9±10.1 | 0.9 |
| BMI, kg/m2 | 21.9±5.4 | 22.7±5.3 | 0.44 |
| CAT score | 15.6±5.3 | 15.3±5.9 | 0.76 |
| Post-BD FEV1, % of the predicted | 50.9±15 | 41.3±11.9 | <0.01* |
| Post-BD sRaw, kPa/sec | 2.75±1.54 | 4.51±3.01 | <0.01* |
| Post-BD RV/TLC, % predicted | 153.1±22.8 | 167.9±29.2 | <0.01* |
| Post-BD TLC | 5.76±1.06 | 5.41±1.18 | 0.08 |
| DLCOc, mmol/min/kPa | 4.83±1.60 | 4.76±1.45 | 0.79 |
| Post-BD R5, cm H2O/L/sec | 5.27±1.30 | 6.75±.29 | <0.01* |
| Post-BD X5, cm H2O/L/sec | -3.02±0.92 | -6.16±2.01 | <0.01* |
| Post-BD R5-19, cm H2O/L/sec | 1.48±0.75 | 2.08±0.82 | <0.01* |
| Post-BD R19, cm H2O/L/sec | 3.44±1.02 | 4.27±0.92 | <0.01* |
| 6MWD, m | 372.7±75.3 | 361±73.5 | 0.39 |
| Desaturation during 6MWT, % | 4.65±3.6 | 5.8±4.4 | 0.11 |
| Change in the Borg score after 6MWT | 2.32±1.75 | 3.0±1.62 | 0.03* |
EFLT: expiratory flow limitation at tidal breath; COPD: chronic obstructive pulmonary disease; BMI: body mass index; CAT: COPD assessment test; Post-BD: post-bronchodilator, parameters measured after 400 μg inhalation of salbutamol; FEV1: forced expiratory volume 1 second; sRaw: specific airway resistance; RV/TLC: ratio of residual volume to total lung capacity; TLC: total lung capacity; DLCOc: hemoglobin adjusted diffusing capacity of the lungs for carbon monoxide; R5: respiratory system resistance at 5 Hz; X5: respiratory system reactance at 5 Hz; R5-19: difference in whole-breath resistance at 5 and 19 Hz; R19: respiratory system resistance at 19 Hz; 6MWD: 6-min walk distance; 6MWT: 6-min walk test.
Table 5.
| Variable | Baseline visit | Follow-up visit | p-value |
|---|---|---|---|
| Post-BD FEV1, L | 1.24±0.49 | 1.31±0.54 | 0.07 |
| Post-BD FVC, L | 2.33±0.60 | 2.32±0.62 | 0.88 |
| RV/TLC, % predicted | 148.7±29.6 | 145.1±36.5 | 0.35 |
| DLCOc, % predicted | 5.27±1.65 | 4.97±1.42 | 0.07 |
| Post-BD R5 | 5±1.68 | 4.58±1.76 | 0.02* |
| Post-BD X5 | -2.88±1.60 | -2.80±1.84 | 0.74 |
| Post-BD ∆X5 | 1.51±1.48 | 1.57±1.85 | 0.79 |
| Post-BD R5-19 | 1.08±0.83 | 1.11±0.82 | 0.79 |
| Post-BD R19 | 3.52±1.13 | 3.19±1.08 | 0.05* |
| CAT score | 13.1±5.2 | 11.3±5.0 | 0.05* |
| 6MWD, m | 384.1±72.6 | 377.2±64.2 | 0.38 |
Post-BD: post-bronchodilator; FEV1: forced expiratory volume 1 second; FVC: forced vital capacity; RV/TLC: ratio of residual volume to total lung capacity; DLCOc: hemoglobin adjusted diffusing capacity of the lungs for carbon monoxide; R5: respiratory system resistance at 5 Hz; X5: respiratory system reactance at 5 Hz; ΔX5: difference in inspiratory and expiratory reactance at 5 Hz; R5-19: difference in whole-breath resistance at 5 and 19 Hz; R19: respiratory system resistance at 19 Hz; CAT: COPD assessment test; 6MWD: 6-minute walk distance.
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 925 View
- 93 Download
- ORCID iDs
-
Amit K. Rath

https://orcid.org/0000-0003-0834-9234 - Related articles
-
What Single Cell RNA Sequencing Has Taught Us about Chronic Obstructive Pulmonary Disease
Proposed Etiotypes for Chronic Obstructive Pulmonary Disease: Controversial Issues


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Data Sharing Statement
Data Sharing Statement Full text via DOI
Full text via DOI Print
Print Download Citation
Download Citation



