 |
 |
| Tuberc Respir Dis > Volume 86(4); 2023 > Article |
|
Abstract
Background
Methods
Results
Conclusion
Notes
Authors’ Contributions
Conceptualization: Choi YJ, Byun MK. Methodology: Park HJ, Cho JH. Formal analysis: Choi YJ, Park HJ, Cho JH, Byun MK. Data curation: Park HJ, Cho JH. Writing - original draft preparation: Choi YJ, Park HJ, Cho JH, Byun MK. Writing - review and editing: Choi YJ, Park HJ, Cho JH, Byun MK. Approval of final manuscript: all authors.
Supplementary Material
Supplementary Table S1.
Fig. 2.
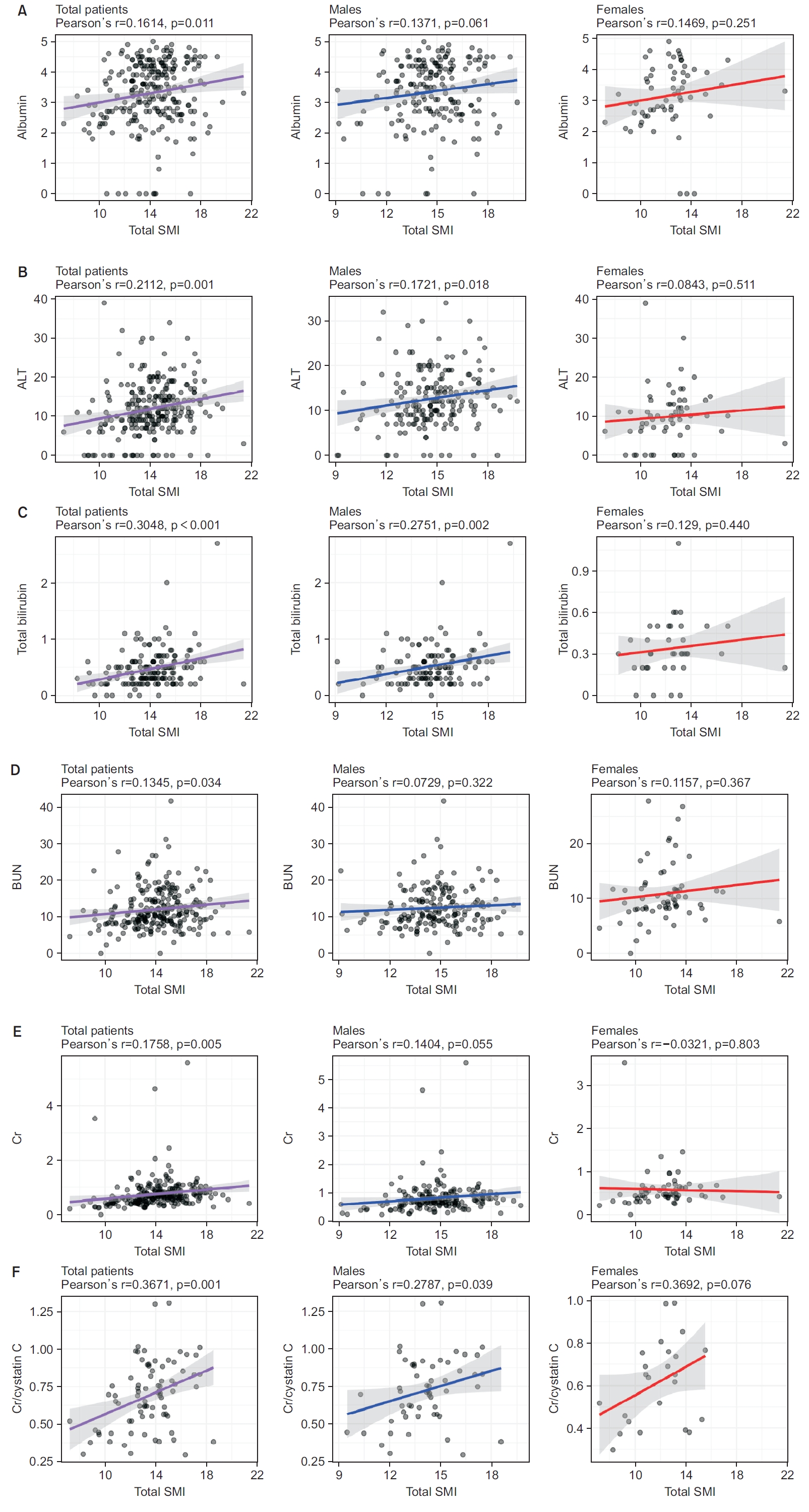
Table 1.
| Group | Total (n=253) | Male (n=189) | Female (n=64) | p-value | ||
|---|---|---|---|---|---|---|
| Age, yr | 71.0 (64.5-77.1) | 71.7 (65.8-77.0) | 68.5 (62.9-79.1) | 0.321 | ||
| Height, cm | 164.1±7.9 | 167.1±6.0 | 155.2±5.9 | <0.001* | ||
| Weight, kg | 61.0 (54.1-68.0) | 63.0 (57.8-69.9) | 54.2 (44.5-62.2) | <0.001* | ||
| BMI, kg/m2 | 22.8 (20.4-25.0) | 22.9 (20.8-24.9) | 22.4 (19.6-25.4) | 0.270 | ||
| Fat mass index, kg/m2 | 6.0 (4.5-8.0) | 5.8 (4.2-7.5) | 7.0 (5.1-10.2) | 0.002* | ||
| Fat free mass index, kg/m2 | 16.4±2.2 | 17.0±2.0 | 14.9±1.9 | <0.001* | ||
| SMI, kg/m2 | 14.2 (12.7-15.5) | 14.7 (13.6-15.9) | 12.5 (10.8-13.2) | <0.001* | ||
| TSMI | 7.4 (6.7-8.1) | 7.6 (7.1-8.3) | 6.7 (5.7-7.2) | <0.001* | ||
| ASMI | 6.8 (6.0-7.4) | 7.1 (6.5-7.7) | 5.7 (4.9-6.1) | <0.001* | ||
| Waist hip ratio | 0.9 (0.9-1.0) | 0.9 (0.9-1.0) | 0.9 (0.8-1.0) | 0.668 | ||
| Comorbidities | ||||||
| CCI score (points) | 4.0 (3.0-7.0) | 4.0 (3.0-6.0) | 5.0 (3.0-7.5) | 0.190 | ||
| Myocardial infarction | 20 (7.9) | 17 (9.0) | 3 (4.7) | 0.403 | ||
| Congestive heart failure | 80 (31.6) | 55 (29.1) | 25 (39.1) | 0.185 | ||
| Peripheral vascular disease | 55 (21.7) | 43 (22.8) | 12 (18.8) | 0.620 | ||
| Cerebrovascular disease | 91 (36.0) | 68 (36.0) | 23 (35.9) | 1.000 | ||
| Dementia | 48 (19.0) | 29 (15.3) | 19 (29.7) | 0.019* | ||
| Chronic pulmonary disease | 253 (100.0) | 189 (100.0) | 64 (100.0) | 1.000 | ||
| Rheumatic disease | 7 (2.8) | 6 (3.2) | 1 (1.6) | 0.811 | ||
| Peptic ulcer disease | 63 (24.9) | 41 (21.7) | 22 (34.4) | 0.063 | ||
| Mild liver disease | 41 (16.2) | 28 (14.8) | 13 (20.3) | 0.404 | ||
| DM without chronic complication | 99 (39.1) | 75 (39.7) | 24 (37.5) | 0.872 | ||
| DM with chronic complication | 46 (18.2) | 36 (19.0) | 10 (15.6) | 0.670 | ||
| Hemiplegia or paraplegia | 49 (19.4) | 34 (18.0) | 15 (23.4) | 0.441 | ||
| Renal disease | 54 (21.3) | 42 (22.2) | 12 (18.8) | 0.682 | ||
| Any malignancy* | 81 (32.0) | 58 (30.7) | 23 (35.9) | 0.533 | ||
| Moderate or severe liver disease | 2 (0.8) | 1 (0.5) | 1 (1.6) | 1.000 | ||
| Metastatic solid tumor | 16 (6.3) | 9 (4.8) | 7 (10.9) | 0.145 | ||
| AIDS/HIV | 0 | 0 | 0 | 1.000 | ||
| History of osteoporosis | 59 (23.3) | 44 (23.3) | 15 (23.4) | 1.000 | ||
| Laboratory data | ||||||
| Protein | 5.9 (4.9-6.8) | 6.0 (5.0-6.9) | 5.5 (4.8-6.6) | 0.154 | ||
| Albumin | 3.4 (2.7-4.2) | 3.5 (2.7-4.2) | 3.2 (2.6-3.9) | 0.120 | ||
| Aspartate aminotransferase | 17.0 (14.0-22.0) | 17.5 (14.0-22.0) | 17.0 (13.0-19.0) | 0.160 | ||
| Alanine aminotransferase | 11.0 (8.0-15.0) | 12.0 (9.0-16.0) | 10.0 (6.0-13.0) | 0.002* | ||
| Total bilirubin | 0.4 (0.3-0.6) | 0.5 (0.3-0.6) | 0.3 (0.2-0.5) | 0.002* | ||
| Blood urea nitrogen | 10.9 (8.5-14.7) | 11.5 (8.9-14.8) | 9.7 (8.1-11.8) | 0.009* | ||
| Creatinine | 0.7 (0.5-0.9) | 0.7 (0.6-0.9) | 0.5 (0.4-0.6) | <0.001* | ||
| Cystatin C | 1.2 (0.9-1.5) | 1.2 (1.0-1.5) | 1.0 (0.9-1.4) | 0.201 | ||
| Creatinine to cystatin C ratio | 0.7±0.2 | 0.7±0.2 | 0.6±0.2 | 0.047* | ||
| C-reactive protein | 1.1 (0.5-2.8) | 1.1 (0.5-3.3) | 1.2 (0.5-2.2) | 0.627 | ||
| Purpose of a BIA test | 0.147 | |||||
| Rehabilitation program | 132 (52.2) | 98 (51.9) | 34 (53.1) | 0.860 | ||
| Sarcopenia screening | 79 (31.2) | 65 (34.4) | 14 (21.9) | 0.062 | ||
| Body fluid evaluation | 4 (1.6) | 3 (1.6) | 1 (1.6) | 0.989 | ||
| Endocrine disorders evaluation | 26 (10.3) | 15 (7.9) | 11 (17.2) | 0.035* | ||
| Health examination | 12 (4.7) | 8 (4.2) | 4 (6.2) | 0.512 | ||
| History of previous AE COPD | 59 (23.3) | 44 (23.3) | 15 (23.4) | 1.000 | ||
BMI: body mass index; SMI: skeletal muscle mass index; TSMI: trunk muscle mass index; ASMI: appendicular muscle mass index; CCI: Charlson comorbidity index; DM: diabetes mellitus; AIDS: acquired immunodeficiency syndrome; HIV: human immunodeficiency virus; BIA: bioelectrical impedance analysis; AE: acute exacerbation; COPD: chronic obstructive pulmonary disease.
Table 2.
| Variable | 1st tertile | 2nd tertile | 3rd tertile | p-value | ||
|---|---|---|---|---|---|---|
| Total patients | 84 | 83 | 86 | |||
| All-cause in-hospital mortality† | 12 (14.3) | 10 (12.0) | 1 (1.2) | 0.006* | ||
| AE COPD within 1 year | 7 (8.3) | 2 (2.4) | 4 (4.7) | 0.216 | ||
| 1 event/yr | 2 (2.4) | 1 (1.2) | 0 | |||
| 2 events/yr | 1 (1.2) | 1 (1.2) | 1 (1.2) | |||
| Above 3 events/yr | 4 (4.8) | 0 | 3 (3.5) | |||
| Pneumonia within 1 year | 4 (4.8) | 3 (3.6) | 2 (2.3) | 0.692 | ||
| 1 event/yr | 1 (1.2) | 1 (1.2) | 0 | |||
| 2 events/yr | 0 | 0 | 1 (1.2) | |||
| Above 3 events/yr | 3 (3.6) | 2 (2.4) | 1 (1.2) | |||
| ER visits within 1 year | 9 (10.7) | 3 (3.6) | 2 (2.3) | 0.037* | ||
| 1 event/yr | 4 (4.8) | 2 (2.4) | 1 (1.2) | |||
| 2 events/yr | 1 (1.2) | 0 | 0 | |||
| Above 3 events/yr | 4 (4.8) | 1 (1.2) | 1 (1.2) | |||
| Admissions within 1 year | 17 (20.2) | 10 (12.0) | 11 (12.8) | 0.259 | ||
| 1 event/yr | 5 (6.0) | 4 (4.8) | 1 (1.2) | |||
| 2 events/yr | 0 | 1 (1.2) | 5 (5.8) | |||
| Above 3 events/yr | 12 (14.3) | 5 (6.0) | 5 (5.8) | |||
| ICU admissions within 1 year | 2 (2.4) | 2 (2.4) | 1 (1.2) | 0.800 | ||
| Male | 63 | 62 | 64 | |||
| All-cause in-hospital mortality† | 9 (14.3) | 8 (12.9) | 1 (1.6) | 0.027* | ||
| AE COPD within 1 year | 6 (9.5) | 1 (1.6) | 4 (6.2) | 0.165 | ||
| Pneumonia within 1 year | 4 (6.3) | 1 (1.6) | 2 (3.1) | 0.358 | ||
| ER visits within 1 year | 9 (14.3) | 3 (4.8) | 2 (3.1) | 0.036* | ||
| Admissions within 1 year | 13 (20.6) | 6 (9.7) | 9 (14.1) | 0.221 | ||
| ICU admissions within 1 year | 2 (3.2) | 0 | 1 (1.6) | 0.365 | ||
| Female | 21 | 21 | 22 | |||
| All-cause in-hospital mortality† | 3 (14.3) | 2 (9.5) | 0 | 0.205 | ||
| AE COPD within 1 year | 1 (4.8) | 1 (4.8) | 0 | 0.582 | ||
| Pneumonia within 1 year | 0 | 2 (9.5) | 0 | 0.121 | ||
| ER visits within 1 year | 0 | 0 | 0 | 1.000 | ||
| Admissions within 1 year | 4 (19.0) | 4 (19.0) | 2 (9.1) | 0.581 | ||
| ICU admissions within 1 year | 0 | 2 (9.5) | 0 | 0.121 | ||
Table 3.
| Variable |
Exacerbation, /yr |
Pneumonia, /yr |
ER visit, /yr |
Admission, /yr |
|||||
|---|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | ||
| Total patients | |||||||||
| Age | -0.095 | 0.132 | 0.008 | 0.899 | 0.043 | 0.500 | 0.093 | 0.140 | |
| CCI | 0.045 | 0.474 | -0.026 | 0.681 | 0.168 | 0.007* | 0.135 | 0.032* | |
| Total SMI | -0.117 | 0.064 | -0.069 | 0.275 | -0.084 | 0.185 | -0.132 | 0.037* | |
| TSMI | -0.127 | 0.043* | -0.064 | 0.314 | -0.106 | 0.092 | -0.111 | 0.079 | |
| ASMI | -0.095 | 0.132 | -0.067 | 0.290 | -0.055 | 0.387 | -0.138 | 0.029* | |
| Albumin | -0.114 | 0.073 | -0.100 | 0.116 | -0.143 | 0.024* | -0.143 | 0.024* | |
| ALT | -0.017 | 0.787 | -0.052 | 0.412 | -0.097 | 0.125 | -0.107 | 0.091 | |
| Total bilirubin | -0.071 | 0.374 | -0.031 | 0.694 | -0.007 | 0.926 | -0.120 | 0.129 | |
| BUN | -0.103 | 0.104 | -0.064 | 0.312 | -0.043 | 0.501 | -0.091 | 0.150 | |
| Creatinine | -0.067 | 0.295 | -0.054 | 0.398 | -0.049 | 0.445 | 0.342 | <0.001* | |
| Cr/Cys C | -0.149 | 0.189 | 0.077 | 0.499 | -0.115 | 0.311 | -0.085 | 0.457 | |
| Male | |||||||||
| Age | -0.111 | 0.130 | -0.015 | 0.843 | 0.045 | 0.538 | 0.029 | 0.692 | |
| CCI | 0.070 | 0.335 | -0.082 | 0.259 | 0.214 | 0.003* | 0.157 | 0.031* | |
| Total SMI | -0.198 | 0.006* | -0.130 | 0.074 | -0.178 | 0.014* | -0.091 | 0.213 | |
| TSMI | -0.196 | 0.007* | -0.117 | 0.108 | -0.191 | 0.008* | -0.089 | 0.222 | |
| ASMI | -0.172 | 0.018* | -0.123 | 0.091 | -0.141 | 0.053 | -0.080 | 0.277 | |
| Albumin | -0.132 | 0.071 | -0.084 | 0.252 | -0.175 | 0.017* | -0.184 | 0.012* | |
| ALT | -0.035 | 0.630 | -0.072 | 0.327 | -0.140 | 0.055 | -0.081 | 0.269 | |
| Total bilirubin | -0.096 | 0.293 | -0.053 | 0.561 | -0.031 | 0.734 | -0.130 | 0.152 | |
| BUN | -0.125 | 0.090 | -0.034 | 0.648 | -0.065 | 0.379 | -0.107 | 0.147 | |
| Creatinine | -0.087 | 0.235 | -0.048 | 0.514 | -0.076 | 0.298 | 0.275 | <0.001* | |
| Cr/Cys C | -0.202 | 0.140 | -0.036 | 0.795 | -0.178 | 0.193 | -0.068 | 0.621 | |
| Female | |||||||||
| Age | -0.131 | 0.302 | 0.070 | 0.583 | NA | NA | 0.189 | 0.134 | |
| CCI | -0.110 | 0.385 | 0.140 | 0.271 | NA | NA | 0.115 | 0.366 | |
| Total SMI | -0.175 | 0.166 | 0.038 | 0.767 | NA | NA | -0.150 | 0.237 | |
| TSMI | -0.183 | 0.149 | 0.038 | 0.768 | NA | NA | -0.102 | 0.421 | |
| ASMI | -0.154 | 0.225 | 0.035 | 0.783 | NA | NA | -0.192 | 0.129 | |
| Albumin | -0.120 | 0.348 | -0.157 | 0.220 | NA | NA | -0.104 | 0.416 | |
| ALT | -0.034 | 0.792 | -0.009 | 0.946 | NA | NA | -0.127 | 0.323 | |
| Total bilirubin | -0.032 | 0.851 | 0.999 | 0.999 | NA | NA | -0.153 | 0.360 | |
| BUN | -0.136 | 0.288 | -0.173 | 0.176 | NA | NA | -0.068 | 0.594 | |
| Creatinine | -0.105 | 0.413 | -0.094 | 0.463 | NA | NA | 0.625 | <0.001* | |
| Cr/Cys C | -0.345 | 0.099 | 0.401 | 0.052 | NA | NA | -0.099 | 0.644 | |
Table 4.
| Variable |
Univariate |
Multivariate† |
VIF | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age, yr | 0.983 (0.939-1.029) | 0.459 | 1.071 (0.960-1.195) | 0.219 | 1.156 |
| Sex (vs. female) | 1.856 (0.411-8.373) | 0.421 | 41.283 (1.203-1,416.885) | 0.039* | 1.479 |
| CCI (score) | 1.088 (0.928-1.274) | 0.298 | 0.890 (0.607-1.303) | 0.548 | 1.348 |
| History of osteoporosis | 0.861 (0.191-3.887) | 0.846 | 0.945 (0.124-7.206) | 0.956 | 1.103 |
| Previous AE COPD | 1.408 (0.434-4.574) | 0.569 | 3.304 (0.427-25.548) | 0.252 | 1.277 |
| Total SMI | 0.807 (0.637-1.023) | 0.077 | |||
| TSMI | 0.615 (0.385-0.982) | 0.042* | 0.200 (0.048-0.838) | 0.028* | 2.187 |
| ASMI | 0.733 (0.471-1.141) | 0.169 | |||
| BMI | 0.881 (0.760-1.022) | 0.095 | 0.946 (0.747-1.197) | 0.644 | 1.646 |
| Protein | 0.709 (0.452-1.110) | 0.133 | |||
| Albumin | 0.724 (0.472-1.111) | 0.139 | |||
| Aspartate aminotransferase | 0.963 (0.848-1.093) | 0.555 | |||
| Alanine aminotransferase | 0.977 (0.898-1.063) | 0.587 | |||
| Total bilirubin | 0.125 (0.004-4.123) | 0.243 | |||
| Blood urea nitrogen | 0.998 (0.901-1.106) | 0.968 | |||
| C-reactive protein | 0.965 (0.836-1.113) | 0.624 | |||
| Creatinine | 0.988 (0.354-2.762) | 0.982 | |||
| Cystatin C | 3.375 (1.372-8.299) | 0.008* | 4.990 (1.070-23.278) | 0.031* | 1.188 |
| Creatinine/cystatin C | 0.071 (0.002-3.165) | 0.172 | |||
† In the multivariate analysis, we included variables known to influence exacerbation and muscle mass measurements, such as age, sex, Charlson comorbidity index, osteoporosis, previous AE COPD, and BMI, along with variables with a univariate p-value of <0.05.
COPD: chronic obstructive pulmonary disease; HR: hazard ratio; CI: confidence interval; VIF: variance inflation factor; CCI: Charlson comorbidity index; AE: acute exacerbation; SMI: skeletal muscle mass index; TSMI: trunk muscle mass index; ASMI: appendicular muscle mass index; BMI: body mass index.
REFERENCES
- TOOLS
-
METRICS

- ORCID iDs
-
Yong Jun Choi

https://orcid.org/0000-0002-6114-2059Min Kwang Byun

https://orcid.org/0000-0003-1525-1745 - Related articles
-
What Single Cell RNA Sequencing Has Taught Us about Chronic Obstructive Pulmonary Disease
Economic Burden of Chronic Obstructive Pulmonary Disease: A Systematic Review



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Data Sharing Statement
Data Sharing Statement Full text via DOI
Full text via DOI Supplement
Supplement Print
Print Download Citation
Download Citation



